Current Suspected Overdose Deaths in Delaware for 2024: Get Help Now!
Find school water testing results and additional resources
Attention Medicaid Participants: Eligibility Renewals Restarted April 1, 2023
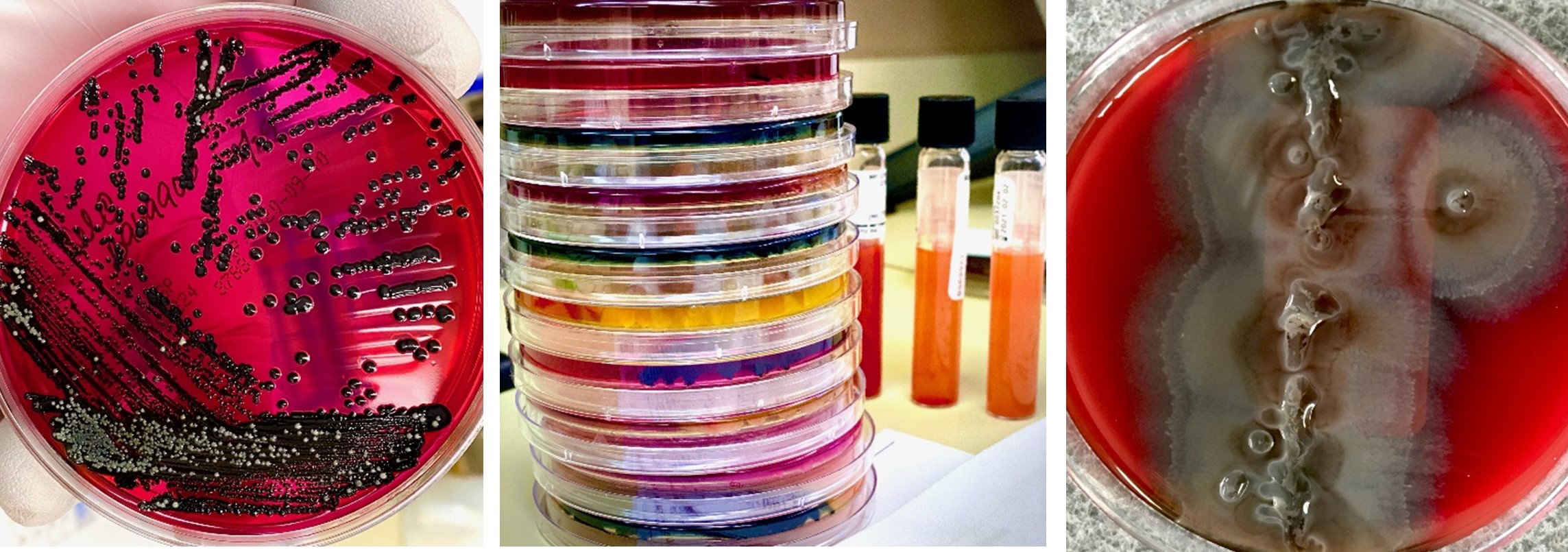
The Microbiology department receives a variety of clinical specimens and bacterial isolates for the purpose of identifying and determining antimicrobial sensitivity profiles of pathogenic microorganisms:
Pathogenic bacteria reported include, but are not limited to, Group A Streptococcus, Staphylococcus aureus, Enterococcus spp., Neisseria gonorrhoeae, Neisseria meningitides, and gram-negative rods including various Enterobacteriaceae and non-Enterobacteriaceae (i.e. Pseudomonas aeruginosa, and Acinetobacter species. DPHL serves clients throughout the state of Delaware, including state service centers, colleges/universities, school-based wellness centers, Department of Corrections (DOC), and partners at the Office of State Medical Examiner's (OCME) office.
Along with the test methods listed above, any suspected pathogens of interest may also be individually requested for screening. The State of Delaware requires all laboratories to submit organisms or clinical samples identified with foodborne disease to the State Laboratory for outbreak surveillance testing. Most laboratories use culture independent diagnostic testing (CIDT) to identify these organisms and no longer require culture for identification. Outbreak surveillance testing requires cultured organisms for testing. If a laboratory or clinic identifies any organisms on the reportable disease list please submit to Delaware Public Health Laboratory.
Return to the Genomic Identification and Surveillance page
Return to the Delaware Public Health Laboratory home page
This page was last updated 4/24
![]() Please note: Some of the files available on this page are in Adobe PDF format which requires Adobe Acrobat Reader. A free copy of Adobe Acrobat Reader can be downloaded directly from Adobe . If you are using an assistive technology unable to read Adobe PDF, please either view the corresponding text only version (if available) or visit Adobe's Accessibility Tools page.
Please note: Some of the files available on this page are in Adobe PDF format which requires Adobe Acrobat Reader. A free copy of Adobe Acrobat Reader can be downloaded directly from Adobe . If you are using an assistive technology unable to read Adobe PDF, please either view the corresponding text only version (if available) or visit Adobe's Accessibility Tools page.