Current Suspected Overdose Deaths in Delaware for 2024: Get Help Now!
Find school water testing results and additional resources
Attention Medicaid Participants: Eligibility Renewals Restarted April 1, 2023
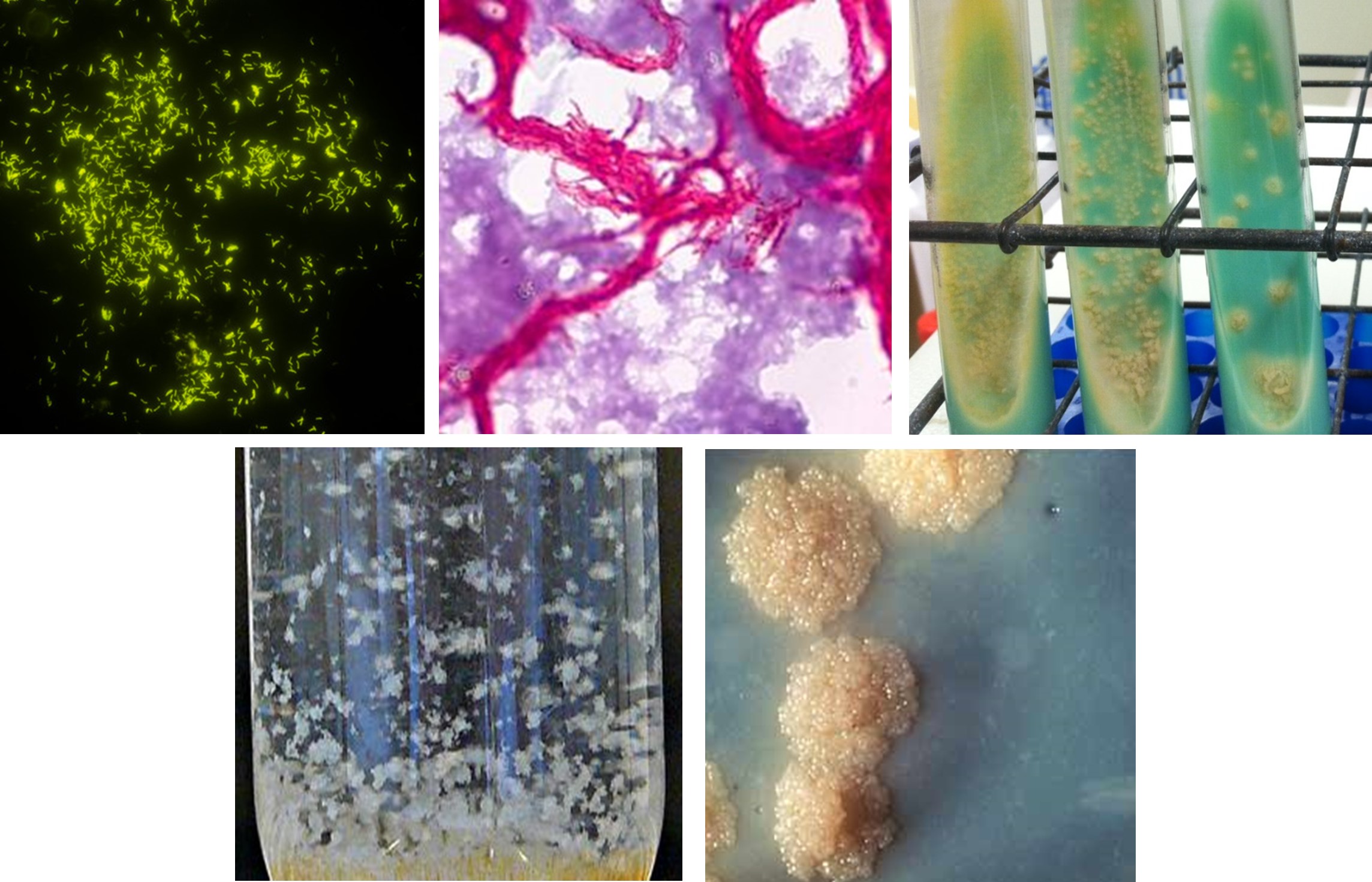
Most Mycobacterial species are environmental or zoonotic in origin, that are poorly transmitted and cause infrequent infections. However, Mycobacterium tuberculosis complex (MTBC), the agent responsible for tuberculosis (TB) disease, is the outstanding exception, as humans are the primary host and reservoir. Despite being both a preventable and treatable disease, each year millions of people worldwide are infected with and succumb to TB. MTBC has become a major contributor to antimicrobial resistance and is a leading cause of death of people with HIV. See Tuberculosis (TB)
The Delaware Public Health Laboratory (DPHL) collaborates with the Division of Public Health TB Elimination Program and State TB clinics to support testing for the identification and antibiotic sensitivity testing of clinical specimens for MTBC. Federal funding has been provided since the early 90's to assist states in this activity. DPHL's Microbiology department operates one of the few biosafety level three (BSL3) laboratories in the state, to support the high-risk culture testing from a variety of clinical sources
Clinical sources include:
DPHL works closely with state, regional, and national partners to provide the most advanced laboratory methods. DPHL utilize specialized broth and liquid selective mediums for cultivation of acid-fast bacilli (AFB). AFB include Mycobacteria, and few other selective organisms. From there, complex laboratory techniques are performed to speciate and determine drug susceptibility.
Available testing includes:
Beyond growth-based laboratory testing, DPHL plans to incorporate whole genome sequencing (WGS) for outbreak surveillance in 2024. WGS is an invaluable tool for TB response. WGS data can be generated and reported faster than growth-based methods, can detect antimycobacterial resistance beyond first line drugs (rifampin, isoniazid, ethambutol, and pyrazinamide), and enables public health to identify potentially related cases through phylogenetic inference.
To assist in preventing spread of TB, DPHL has performed Interferon gamma release assay (IGRA) since 2009. By identifying latent TB disease, or asymptomatic individuals infected with TB. DPH can implement therapeutic interventions, reducing onset of active disease, and potential spread to the public. This test is performed using whole blood and requires complex laboratory practice through enzyme linked immunosorbent assay (ELISA). Samples are batched and tested weekly. This has allowed DPH to be effective in reducing the spread of TB in our community.
Tuberculosis is one of the oldest diseases in the recorded history of mankind and it continues to remain one of the deadliest infectious diseases in the world today. Through collaborative efforts with local, regional, and national partners, DPHL strives to work towards a future with increased awareness and reduced infections with TB. See below for further resources for TB testing.
Return to the Genomic Identification and Surveillance main page
Return to the Delaware Public Health Laboratory home page
This page was last updated 4/24
![]() Please note: Some of the files available on this page are in Adobe PDF format which requires Adobe Acrobat Reader. A free copy of Adobe Acrobat Reader can be downloaded directly from Adobe . If you are using an assistive technology unable to read Adobe PDF, please either view the corresponding text only version (if available) or visit Adobe's Accessibility Tools page.
Please note: Some of the files available on this page are in Adobe PDF format which requires Adobe Acrobat Reader. A free copy of Adobe Acrobat Reader can be downloaded directly from Adobe . If you are using an assistive technology unable to read Adobe PDF, please either view the corresponding text only version (if available) or visit Adobe's Accessibility Tools page.