Current Suspected Overdose Deaths in Delaware for 2024: Get Help Now!
Find school water testing results and additional resources
Attention Medicaid Participants: Eligibility Renewals Restarted April 1, 2023
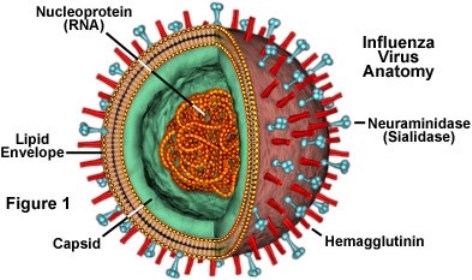
Influenza A and B viruses are responsible for respiratory epidemics each year. Influenza A Viruses are categorized by their surface proteins: Hemagglutinin (H) and Neuraminidase (N). Since Influenza B viruses are structurally different, they are only broken down into two lineages: B/Yamagata and B/Victoria (CDC, 2017).
The Delaware Public Health Lab performs influenza identification, molecular detection, and subtyping. Influenza surveillance is performed as part of the CDC-World Health Organization (WHO) Influenza surveillance effort. This information, along with a select number of isolates, is provided to CDC and the WHO to aid in surveillance efforts and future vaccine formulation.
DPH Flu Immunization Information
Delaware Weekly Influenza Surveillance Reports
CDC Guidelines for Clinicians on the Use of Rapid Influenza Diagnostic Tests
References: Centers for Disease Control and Prevention, 2017. Types of Influenza Viruses.
Return to the Delaware Public Health Laboratory page
This page was last updated 4/2024
![]() Please note: Some of the files available on this page are in Adobe PDF format which requires Adobe Acrobat Reader. A free copy of Adobe Acrobat Reader can be downloaded directly from Adobe . If you are using an assistive technology unable to read Adobe PDF, please either view the corresponding text only version (if available) or visit Adobe's Accessibility Tools page.
Please note: Some of the files available on this page are in Adobe PDF format which requires Adobe Acrobat Reader. A free copy of Adobe Acrobat Reader can be downloaded directly from Adobe . If you are using an assistive technology unable to read Adobe PDF, please either view the corresponding text only version (if available) or visit Adobe's Accessibility Tools page.